Excipients – It’s Good to be Bland!

An estimated 80% of new drug actives are classified as poorly soluble. There are a number of approaches for enhancing solubility – alternative salt forms, particle size reduction and solubility-enhancing excipients, among others. Like APIs, many excipients are known to be bitter or have other aversive sensory attributes including aromatic off-notes (malodor) and trigeminal irritation (e.g., tongue sting and mouth or throat burn). In some cases, the taste masking challenge of the excipient system can be greater than for the API itself.
Excipients, the “inactive” ingredients in drug products, serve many important functions:
- Increase solubility
- Maintain physical, chemical and microbial stability
- Enhance disintegration
- Control release
- Improve manufacturability
- Many others
Without excipients, there would be no dosage forms – just drug actives in bottles!
Flavor Attributes of Excipients
“Flavor” (discussed previously) is a combination of all attributes perceived when a product is in the oral cavity – taste, aroma, mouthfeel and texture. It’s common knowledge that most Active Pharmaceutical Ingredients (APIs) are bitter or have other “negative” or aversive sensory attributes such as an unpleasant aroma (malodor), mouth irritation, or a gritty texture that can adversely affect doing compliance.
Less well known is that many excipients also have aversive sensory attributes – sometimes more than the API– that can unwittingly exacerbate the taste masking challenge.
Sensory Attributes of Selected Excipients
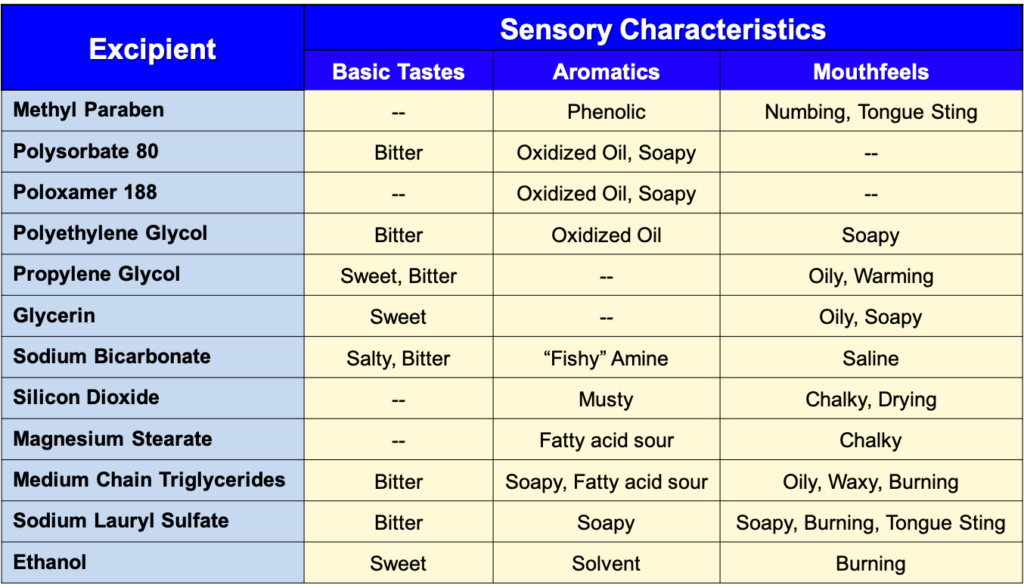
The Effects of Excipients on Palatability
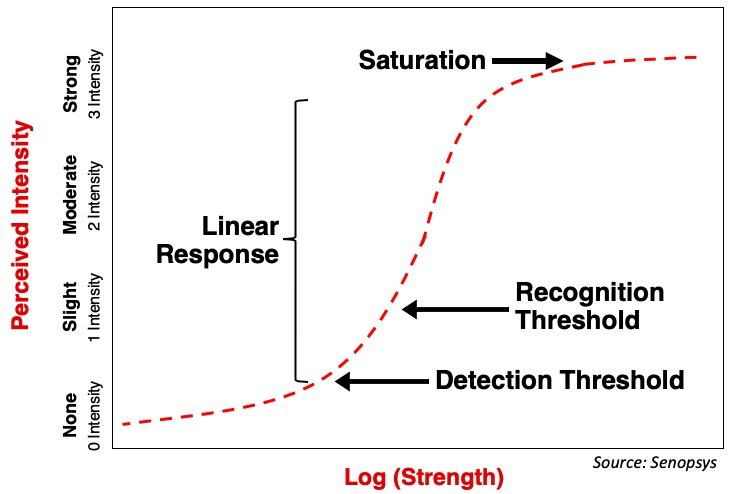
Palatable drug products are those in which the aversive sensory attributes have been minimized or eliminated. In other words, they are not overly bitter, produce little trigeminal irritation, are smooth not gritty and have no perceptible malodors. From a quantitative perspective this means the aversive sensory attributes are below the recognition threshold.
Psychophysical Signal Response Curve
An estimated 80% of new drug actives are classified as poorly soluble. There are a number of approaches for enhancing solubility – alternative salt forms, particle size reduction and solubility-enhancing excipients, among others.
Many of the solubility-enhancing excipients can be particularly challenging from a taste masking perspective – often greater than for the API itself. To illustrate, we analyzed samples of five different classes of solubility enhancers:
- Polysorbate surfactants
- Glyceride emulsifiers
- PEGylated oil surfactant
- Tocopherol surfactant
- Propylene glycol
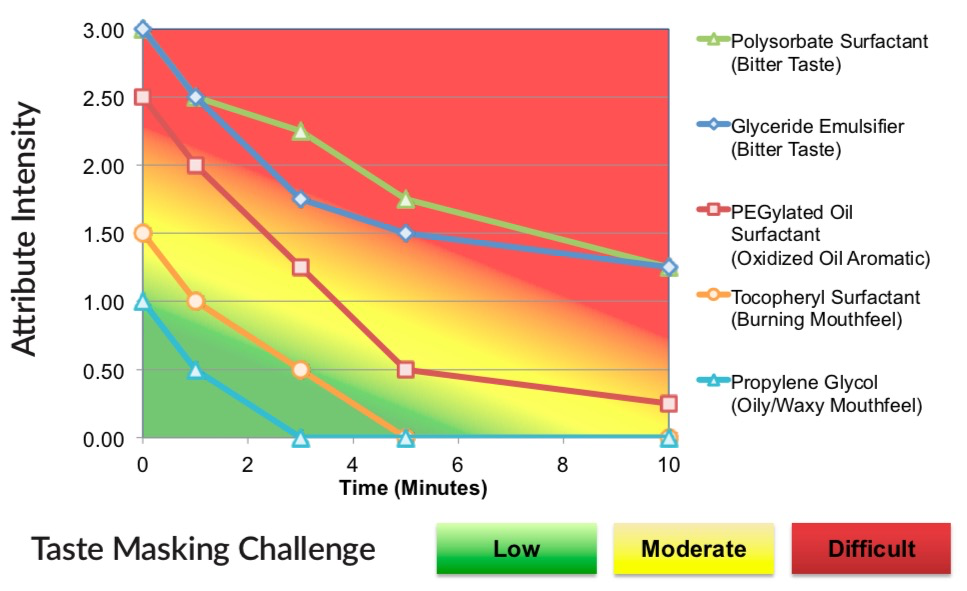
Samples of each were evaluated at their typical usage level using the Flavor Profile Method of sensory analysis. The results are summarized below where the intensity of the strongest negative attribute is plotted as a function of time.
Aversive Attribute Intensity of Five Classes of Solubility Enhancers
Interpreting the Results
As shown, the five classes of solubility enhancing excipients varied in terms of their sensory attributes – most notably bitter basic taste, aromatic off-notes, trigeminal irritation (burning) and mouthfeel effects (oily/waxy).
Difficult Taste Masking Challenge The polysorbate surfactant and glyceride emulsifier represent difficult taste masking challenges, independent of the contribution of the drug active. While the bitterness profiles can be reduced through the application of taste/taste interaction, the resulting bitterness will still be above the recognition threshold.
Moderate Taste Masking Challenge The oxidized oil aromatics of the pegylated oil surfactant represent a moderate-to-difficult taste masking challenge but may be substantially reduced with a properly formulated flavor system.
Low Taste Masking Challenge The burning mouthfeel of the tocopherol surfactant is slightly above the recognition threshold and would not be expected to be perceptible in a final, optimized formulation.
Propylene glycol is comparatively “bland” with the lowest overall Flavor Profile. Unfortunately, each class of solubility enhancers has different performance characteristics and propylene glycol is unlikely to effective for many APIs.
Suppliers Matter
Excipients from different suppliers are often thought to be “interchangeable.” While this may be the case for some functional properties, it is seldom the case for flavor quality.
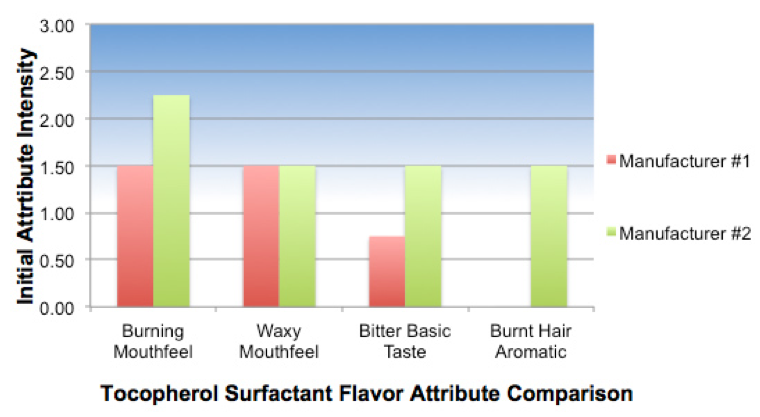
To illustrate, the aversive sensory attributes of the same tocopherol surfactant from two suppliers are shown below. The material from Manufacturer #1 has a “cleaner” Flavor Profile with lower bitterness, mouthfeels (burning and waxy) and absence of malodor (burnt hair). Use of this material will result in a more palatable formulation than from Manufacturer #2.
Flavor Quality Comparison of Tocopherol Surfactant by Supplier
Quality By Design
All drug products contain excipients and formulators need to be cognizant not only of the function of each excipient, but of their sensory characteristics. It is widely under-appreciated until it’s too late that the aversive sensory attributes of many excipients can be greater than the drug active. This is particularly true of solubility enhancers, which are increasingly required for todays intractable APIs.
For this reason, it’s important to consider the flavor effects of candidate excipients early in the formulation development process to avoid creating or exacerbating the taste masking challenge. For example, in conducting design-of-experiments for solubility enhancement, it is important to assess the sensory attributes of the excipient blends in an effort to simultaneously optimize for solubility and taste.
In summary, when it comes to excipients, it’s good to be bland!
Taste Masking Challenge? Senopsys Can Help!
Are you faced with the need to develop a palatable drug product to support clinical trials or commercial development? Our scientists are expert in both taste assessment and taste masking.
We use our experienced GCP-compliant taste panels and analytic tools to quantify the taste masking challenge and guide formulation development. And we apply a structured, sensory-directed development approach pioneered in the food industry to create palatable, taste-masked drug formulations for liquids, powders and solids.

