Palatable Drug Products Defined – It’s More Than Yuck and Yum

Regulations in the United States and European Union are incentivizing (via pediatric exclusivity) and requiring the development of pediatric medicine. These regulations are designed to ensure that every new drug will be evaluated for use in pediatric patients and studied in this population when appropriate.
A key requirement is the submission of detailed plan that outline the studies to be conducted as part of the pharmaceutical and clinical development program.
- EMA – Paediatric Investigation Plan (PIP)
- FDA – Pediatric Study Plan (PSP)
The regulatory agencies require that companies demonstrate the “palatability” of drug products in pediatric patient populations as part of the pharmaceutical and clinical development program (unless a PIP or PSP waiver is granted).
But what is meant by “palatable”?
Defining “Palatable”
There are many definitions of “palatable” – dictionaries, expert opinions and regulatory perspectives.
The European Medicines Agency (EMA) has proposed its own definition:
The challenge with all of these definitions is that they are subjective.
What’s the alternative?
Senopsys has advanced a definition of “palatable” that is based on the scientific principles of perception:
(Senopsys)
A key feature of Senopsys’ definition of palatable is that it is objective, with a measureable endpoint.
Our Sensory World
Psychophysics is the scientific study of the relation between stimulus and perception. Nearly all stimuli (sound, sight, taste, smell) follow a similar response profile idealized below.
Illustrative Stimuli Response Curve
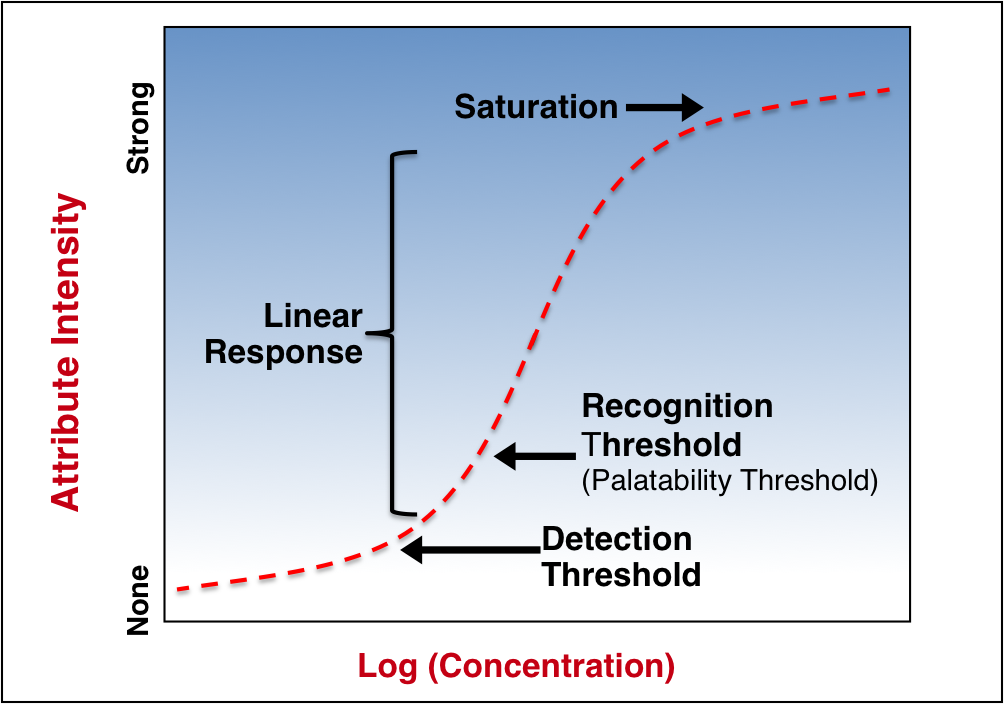
At low and high strengths, perceived changes in stimulus intensity become asymptotic to the change in strength. Thus the linear portion of this stimulus curve defines the human sensory world.
To this response curve, three important cognitive functions can be ascribed:
- Detection threshold
- Recognition threshold
- Saturation
Detection Threshold
Below the detection threshold, stimuli cannot be perceived. At the detection threshold, stimuli can be perceived but not described. In the case of taste, the detection threshold is the point at which a subject would indicate a tasted solution as different than water, but could not describe the difference. In the corollary of sound, a voice can be heard at the detection threshold, but the words are not understood.
Recognition Threshold
The recognition threshold is the strength at which stimuli elicit a qualitative response from the subject, e.g., “sweet”, “cherry”, “cooling” or in the case of sound, a hushed but intelligible whisper.
Saturation
As stimuli strength continues to increase, a point of saturation is reached, beyond which large changes in strength are not perceived as stronger. The analogy to sound is music at a rock concert – beyond a certain level, increases in speaker output only produce pain.
The recognition threshold is the most important in developing palatable drug formulations – aversive sensory attributes below this intensity will not be perceptible to patients.
How is the recognition threshold measured?
Sensory Analysis
In the field of sensory science, human taste panels are used to measure intensity changes in the linear portion of the stimulus response curve. Senopsys uses Flavor Profile, an internationally recognized, open-source (i.e., non-proprietary) method of sensory analysis.
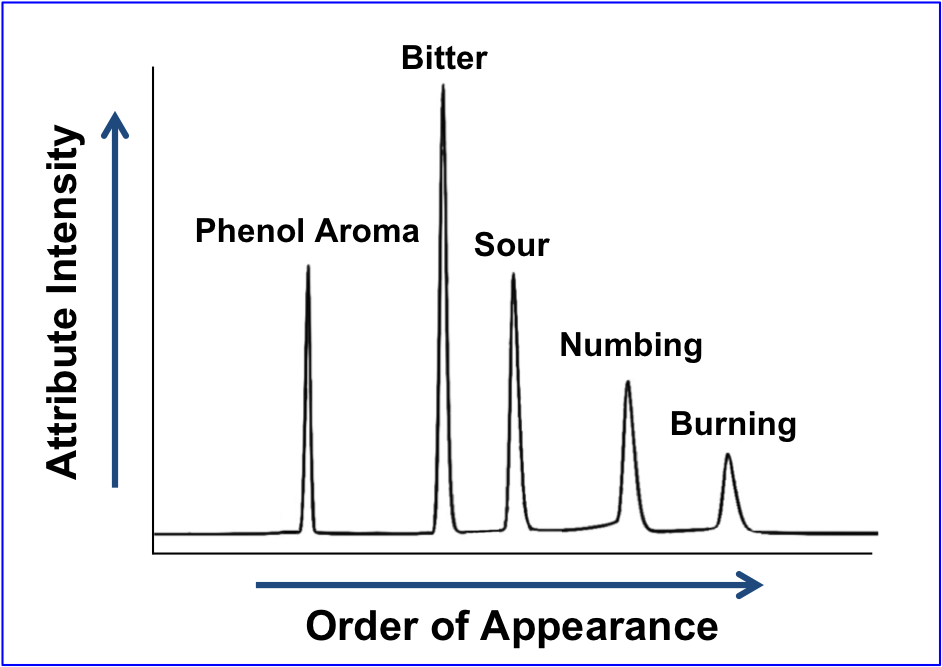
Flavor Profile uses trained adult assessors, known as “panelists” to:
- Identify the perceived attributes – taste, aroma, mouthfeel and texture.
- Rate the intensity of each perceived attribute using a measurement scale that is established with chemical reference standards over the linear portion of the stimulus response curve.
The output is analogous to a chromatogram, wherein each peak represents a separately identified sensory attribute and the peak height/area corresponds to perceived strength or intensity of each attribute.
What is a Palatable Drug Formulation?
Returning to the concept of palatability, many drug actives are extremely bitter or have other aversive attributes such as a malodor, trigeminal irritation, or a gritty texture.
It’s an industry myth that flavor preference – orange, grape, chocolate or mint – is the primary determinant of palatability of drug products. In actuality, the true measure of palatability is the degree to which the aversive attributes have been eliminated.
Understanding the psychophysics of perception, palatable drug are those in which the aversive attributes have been reduced below their recognition thresholds. In other words, they are not overtly bitter, produce little trigeminal irritation, are smooth not gritty and have no perceptible malodors.
Developing Palatable Drug Products
Developing palatable, taste-masked pediatric drug formulations can be a daunting undertaking filled with many clinical regulatory, market and technical challenges. Analytical sensory analysis methods such as Flavor Profile are used to guide formulation development from successful first-in-human studies to clinical trial materials manufacture:
- Initial Taste Assessment – identify and quantify aversive sensory attributes
- Drug Product Design – define Critical Quality Attributes for taste, aroma, mouthfeel and texture
- Formulation Development – guide taste masking applying Quality by Design principles
- Technology Guidance – determine requirements for, and effectiveness of, enabling taste masking technology, e.g., encapsulation, adsorption, compexation
- Stability Testing – measure palatability of formulations on ICH stability
In future posts we’ll describe some of the processes, tools and techniques available to formulation scientists to help them manage the technical risks associated with developing and manufacturing palatable drug products.
- 1[Regulatory perspectives on acceptability testing of dosage forms in children, Piotr Kozarewicz EMA, 5th EuPFI Conference, Sept 2013, and International Journal of Pharmaceutics 469 (2014) 245–248 ].
Taste Masking Challenge? Senopsys Can Help!
Are you faced with the need to develop a palatable drug product to support clinical trials or commercial development? Our scientists are expert in both taste assessment and taste masking.
We use our experienced GCP-compliant taste panels and analytic tools to quantify the taste masking challenge and guide formulation development. And we apply a structured, sensory-directed development approach pioneered in the food industry to create palatable, taste-masked drug formulations for liquids, powders and solids.

