Regulatory Requirements – Pediatric Formulation Development
Regulations in the US and EU both require and incentivize (via pediatric exclusivity) development of pediatric medicines. These regulations are designed to ensure that every new drug will be evaluated for use in pediatric patients and studied in this population when appropriate.
A key requirement is the submission of detailed plans that outline the studies to be conducted as part of the pharmaceutical and clinical development program:
- EMA – Paediatric Investigation Plan (PIP)
- FDA – Pediatric Study Plan (PSP)
The regulatory agencies require that companies demonstrate the “palatability” of drug products in pediatric patient populations as part of the pharmaceutical and clinical development program (unless a PIP or PSP waiver is granted).
Definition of Palatable
Many Active Pharmaceutical Ingredients (API) are extremely bitter or have other aversive sensory attributes such as a malodor, trigeminal irritation, or a gritty texture that can adversely affect doing compliance.
There are many definitions of “palatable” – dictionaries, expert opinions and regulatory perspectives. However, the shortcoming of all of them is that they are subjective.
Senopsys has advanced a definition of “palatable” that is based on the scientific principles of perception:
are below the recognition threshold”
A key feature of Senopsys’ definition of palatable is that it has a measureable endpoint.
The Science of Perception
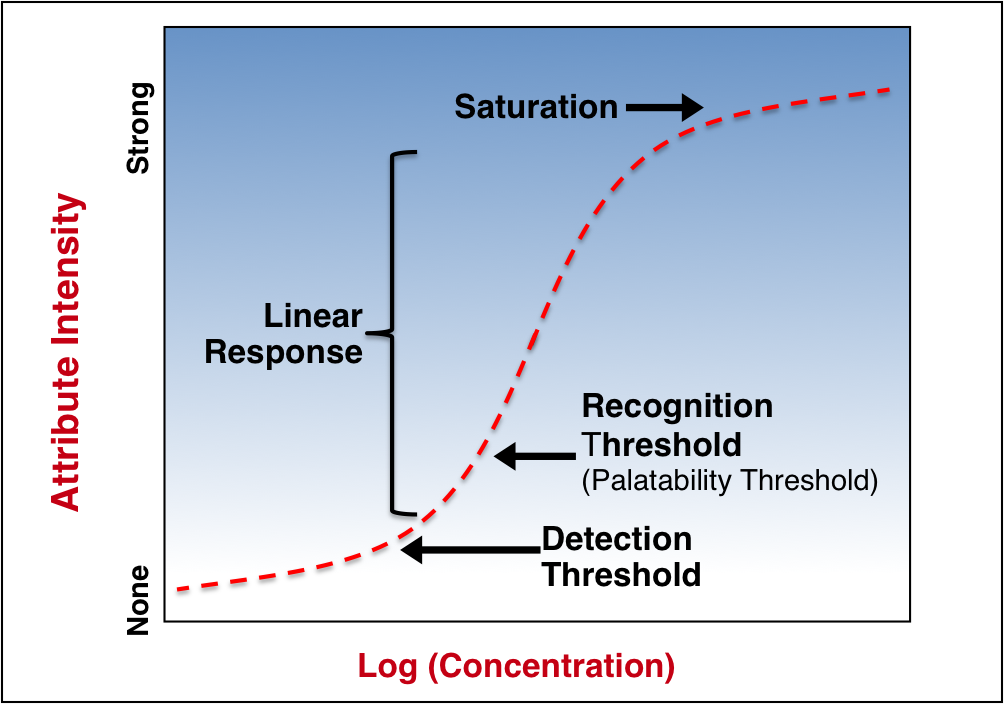
At low and high strengths, perceived changes in stimulus intensity become asymptotic to the changes in strength. Thus the linear portion of the stimulus response curve defines the human sensory world.
To this response curve, three important cognitive functions can be ascribed:
- Detection threshold – strength at which subjects report a difference from baseline but that cannot be described.
- Recognition threshold – strength at which stimuli elicit a qualitative response, e.g., bitter, sweet, cooling, cherry.
- Saturation – point beyond which increases in strength produce no additional response.
The recognition threshold is the most important in developing palatable pediatric drug formulations – aversive sensory attributes below this intensity will not be perceptible to patients.
Illustrative Stimuli Response Curve
Sensory Analysis
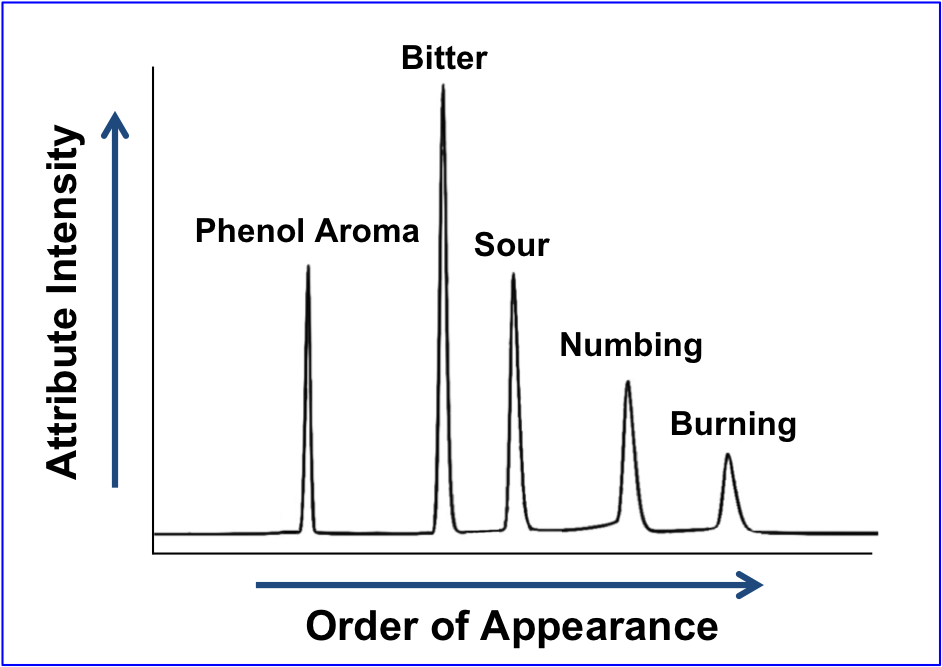
Senopsys uses the seminal Flavor Profile Method to measure the sensory attributes of formulations – taste, aroma, mouthfeel and texture. Flavor Profile is an internationally recognized, open-source method of sensory analysis. The Flavor Profile intensity measurement scale is established with chemical reference standards over the linear portion of the stimulus response curve. The output is analogous to a chromatogram, wherein each peak represents a separately identified sensory attribute and the peak height/area corresponds to its perceived intensity.
Flavor Profile is used to guide the development of taste-masked pediatric drug formulations, i.e. to measure the reduction in the aversive sensory attributes.
Understanding the psychophysics of perception, palatable pediatric drug formulations are those in which the aversive attributes have been reduced below their recognition thresholds. In other words, they are not overtly bitter, produce little trigeminal irritation, are smooth not gritty and have no perceptible malodors.
The Output of Flavor Profile Analysis is Analogous to a Chromatogram