Mini-Tablet Taste Assessment
Challenge:
The client was developing a pediatric dosage form of a poorly soluble API. To improve bioavailability a hot melt extrudate was developed for dosing as a suspension either in foods or in compressed mini-tablets. A preliminary dose-response sensory analysis of aqueous extrudate suspensions indicated a strong intensity lingering bitterness (>30 minutes) across all clinically relevant strengths. To improve palatability, alternative tablet film coatings were explored.
Senopsys Approach:
Senopsys evaluated the taste masking effectiveness of two commercially available coatings via two methods – direct evaluation of mini-tablets and mini-tablets dispersed in model foods.
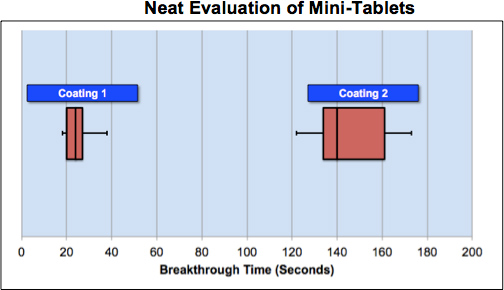
First, as an in-vivo measure of coating performance (dissolution), mini-tablets were rolled neat in the oral cavity. Senopsys’ trained sensory panelists measured the time to reach a moderate intensity (2) bitterness using the Flavor Profile scale. The goal being that the mini-tablet coating should be sufficiently durable to allow patients to swallow the mini-tablets before bitterness is perceived (i.e., before bitter “breakthrough”).
Next, the film-coated mini-tablets were evaluated after stirring into two model “pediatric-friendly” foods (neutral pH and acidic pH). Each were measured as function of hold time in the foods up to 15 minutes to simulate dose preparation and administration time by the parent or caregiver.
Results: Direct (neat) evaluation of dissolution times resulted in marked differences between the two coatings. Bitter breakthrough of the mini-tablets with Coating 2 was six times longer than for Coating 1 (146 vs. 24 seconds). The oral residence time of mini-tablets during normal dose administration is expected to be less than 146 seconds (~2½ minutes). Therefore bitterness is not likely to be perceived for Coating 2. In contrast, it is likely that some patients would perceive bitterness of mini-tablets coated with Coating 1.
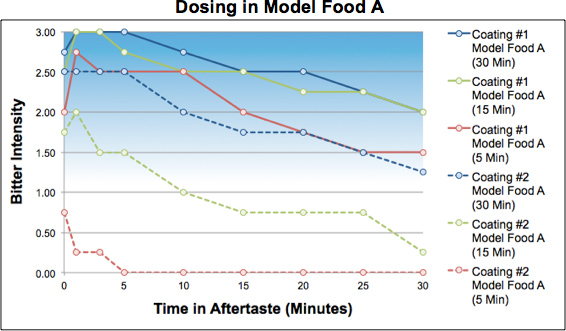
The performance of the two film coatings was also found to be very different in the model foods. Shown below are the results for the two coating variables in the model food products as a function of hold time.
In Model Food A, Coating 2 was more “robust” (lower bitterness) overall than Formulation 1 but was sensitive to hold time in the model food. Only mini-tablets with Coating 2 administered within about 5 minutes of dose preparation would not be perceptibly bitter to patients, i.e., intensity ≤ 1.
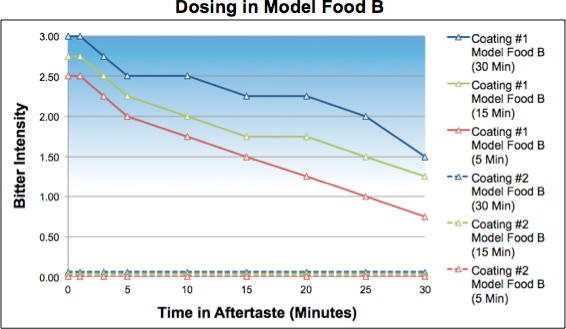
In Model Food B, mini-tablets with Coating 1 produced a strong intensity and lingering bitterness at all time conditions that increased with hold time. In contrast, the mini-tablets with Coating 2 produced no measurable bitterness at any of the hold times.
In summary, trained human taste panels can be used to rapidly assess the taste making effectiveness of tablet coating systems, including their performance in model foods.

