Methods of Analysis Used In Taste Masking
The food industry strives to create products that “delight the palate” as flavor quality drives sales. In contrast, medicines are developed for efficacy and safety. In order to help ensure successful dose administration, drug products need only be palatable. In other words, the bar is somewhat lower for medicines than for foods.
But what is palatable? Many drug substances are bitter, some extremely so, or have other “negative” or aversive sensory characteristics such as an unpleasant aroma or mouth irritation. Palatable drug products are those in which the aversive sensory attributes are below the level of perception.
In other words they are not overly bitter, produce little trigeminal irritation, are smooth not gritty and have no perceptible malodors.
What is Sensory Analysis?
Sensory analysis is defined as a scientific discipline used to evoke, measure, analyze, and interpret those responses to products that are perceived by the senses of sight, smell, touch, taste, and hearing1.
By definition, sensory analysis methods entail the use of human subjects (i.e., in-vivo methods). These methods are most commonly employed to guide the development of palatable formulations.
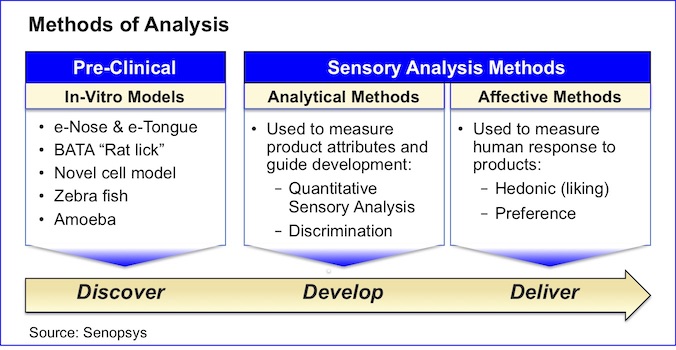
There are two broad classes of sensory analysis methods:
- Analytical methods – used to measure the attributes of products and guide formulation development.
- Affective methods – used to measure human response to products and select the most liked or preferred formulation.
There are also in-vitro models that have been advanced for studying the taste attributes of new chemical entities (NCEs) in preclinical research. As these models do not use human subjects they are not considered sensory analysis methods per se.
Analytical Sensory Analysis Methods
Analytical methods are used to measure the attributes of products.
The two most commonly used analytical techniques in pharmaceutical development are quantitative sensory analysis using trained subjects and discrimination testing using trained or untrained subjects.
The analytical methods are used to identify and quantify the sensory attributes of products – basic taste, aroma, mouthfeel, and texture. These are most commonly employed to quantify the taste challenge and guide the development of palatable, taste-masked formulations.
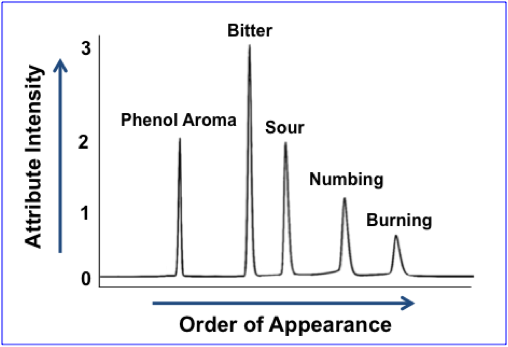
There are several quantitative sensory analysis methods – Senopsys uses Flavor Profile, an internationally recognized, open-source (i.e., non-proprietary) method of sensory analysis. Flavor Profile uses trained adult assessors, known as “panelists” to:
- Identify the perceived attributes – taste, aroma, mouthfeel and texture.
- Rate the intensity of each perceived attribute using a measurement scale that is established with chemical reference standards
The output is analogous to a chromatogram, wherein each peak represents a separately identified sensory attribute and the peak height/area corresponds to perceived strength or intensity of each attribute.
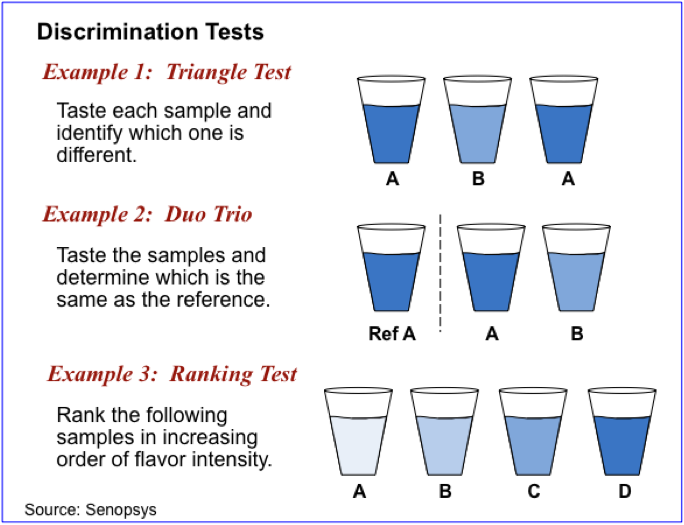
Discrimination testing techniques are used to determine whether there is a detectable difference between two or more formulations. They are frequently used to support ingredient substitution decision and for quality control purposes. Common discrimination tests are shown below.
Affective Sensory Analysis Methods
Affective tests are used to measure human response to products.
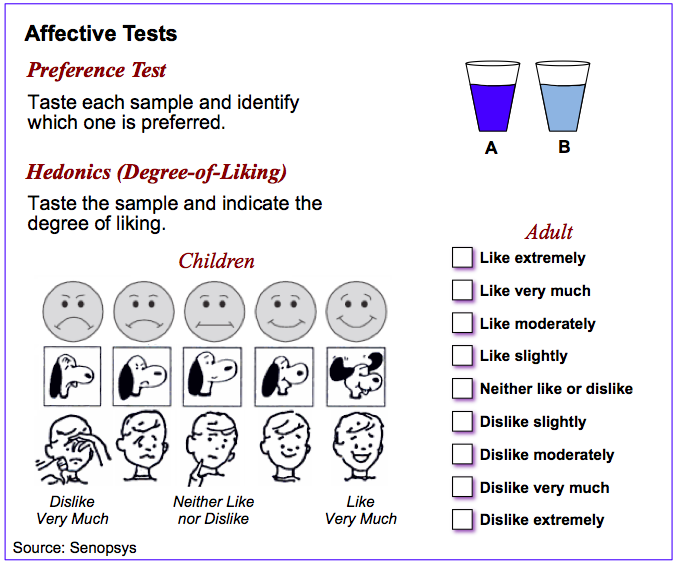
The most commonly used affective tests are hedonics (liking) or preference and these always use untrained respondents, ideally the target patient population. Affective tests can be conducted on either an absolute or relative basis. Liking is measured on an absolute basis whereas preference is always a relative measure. As pre-verbal children and infants are unable to express their preferences clearly, other techniques must be employed. These include caregiver interview questionnaires, facial expression interpretation or Likert-type pictorial hedonic scales.
Picking the Right Method
Due to the unique challenges surrounding drug development, there are a variety of analysis methods that can be used to answer different taste questions along the continuum from pre-clinical assessment to formulation development to manufacture of clinical trial materials and commercial products.
In-vitro models are being studied to gain early insight into potential aversive attributes of drug actives in pre-clinical research. However, there is limited published research that shows a relationship between preclinical tests of NCEs and human response. As such these models are currently of limited utility in predicting taste issues or guiding the development of palatable formulations.
When first-in-human clinical studies indicate potential palatability issues, or if these remain unknown, quantitative sensory analysis methods are commonly used to guide formulation development. For example to:
- Identify the aversive sensory attributes
- Quantify the taste masking challenge
- Measure reduction in aversive taste attributes
- Develop palatable, age-appropriate formulations
For pediatric drug products, the regulatory agencies are increasingly requiring manufacturers to conduct palatability studies in the target patient population to demonstrate ease of dose administration. Affective tests are designed and executed in a clinical setting to measure degree-of-liking or preference as indirect measures of “palatability” or “acceptability.” The design of the test instrument is dependent upon the age of the children as described above.
Finally, in-vitro techniques such as the “electronic tongue” (“e-tongue”) can also be used to measure differences from an established “gold standard.” In the absence of correlation to human sensory response data, these techniques are of limited utility in development environments or selecting formulations and are most commonly used in quality control.
The Developer’s Toolbox
There are many sensory analysis tools available to formulation scientists to help them develop palatable, taste-masked drug products. No single method is amenable to addressing the differing needs along the development pathway. The fundamental choice depends upon whether you want to measure the product’s attributes (analytical methods) or patient response to the product (affective methods). Hopefully this post will help you make the right choice!
-
1Stone H and Sidel J (1988). Sensory Evaluation Practices
Taste Masking Challenge? Senopsys Can Help!
Are you faced with the need to develop a palatable drug product to support clinical trials or commercial development? Our scientists are expert in both taste assessment and taste masking.
We use our experienced GCP-compliant taste panels and analytic tools to quantify the taste masking challenge and guide formulation development. And we apply a structured, sensory-directed development approach pioneered in the food industry to create palatable, taste-masked drug formulations for liquids, powders and solids.

