Measurement of In-vivo Disintegration Times of Orally Disintegrating Tablets
Challenge: Orally disintegrating tablets (ODTs) are an increasingly popular dosage form for children, the elderly and other patients who have difficulty swallowing solid oral dosage forms (dysphagia).
The Nomenclature Standards Committee of the Center for Drug Evaluation and Research (CDER) at the Food and Drug Administration (FDA) developed the following definition for an ODT as a new dosage form in 1998: “A solid dosage form containing medicinal substances which disintegrates rapidly, usually within a matter of seconds, when placed upon the tongue.”
FDA reported a range of in-vitro disintegration times ranged from a few seconds to longer than a minute, with a large majority of products having disintegration times of approximately 30 seconds or less based on the United Sates Pharmacopeia (USP) test method or alternative (Guidance for Industry – Orally Disintegrating Tablets; December 2008). Based on this reported experience, FDA developed the following guidance for solid tablets labeled as orally disintegrating tablets: (1) in-vitro disintegration time of approximately 30 seconds or less and (2) tablet weight not more than 500 mg, unless justified by based on performance (disintegration time).
There are a number of ODT technologies, variously based on lyophilization, spinning disc and compression approaches that utilize proprietary ingredients, processing techniques or both.
Several clients have looked to Senopsys to assist them in developing palatable ODTs.
Senopsys Approach: Senopsys has developed sample evaluation protocols to reproducibly measure in-vivo disintegration time. Rapid disintegration is an important but insufficient criterion in determining the palatability of this dosage form. Other important criteria include texture, mouthfeel, grittiness, and flavor quality (taste masking).
Results: The results of a benchmarking study of commercial product in-vivo disintegration times are shown below. In this study, measured in-vivo disintegration times ranging from a few seconds to over 1.5 minutes, with some technologies producing greater variability than others. As can be seen, some purportedly “fast dissolving” tablets have disintegration times more similar to chewable tablets.
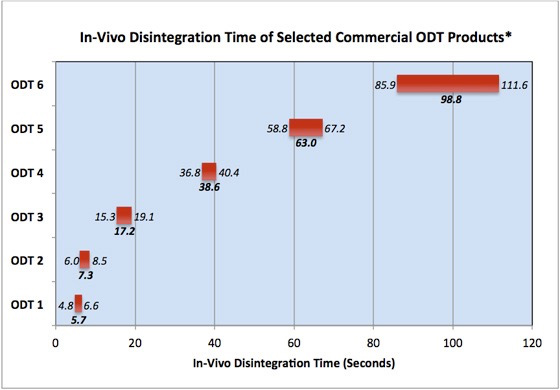
* The range and mean panelist disintegration times are plotted

