Guiding Taste Masking Technology Optimization
Challenge:
The client was interested in developing a palatable orally disintegrating tablet (ODT) form of an approved drug. Senopsys’ initial taste assessment indicated that an adsorption or particle coating technology would be required to develop a palatable ODT due to the strength and duration of multiple negative sensory attributes at a clinically relevant dosage strength (40mg). These aversive attributes included: bitter, sour and salty basic tastes; tongue sting, numbing, tannin and astringent mouthfeels and metallic aromatics, many of which lingered at patient-perceptible levels for 30 minutes. Candidate drug coating technologies for taste masking include API encapsulation or Wurster coating, film formers (e.g. Eudragit or Opadry), hot melt extrusion, ion exchange resins (e.g. Amberlite), and the use of complexation agents (e.g. cyclodextrins). As none of these coating technologies are 100% effective barriers, some free-API will remain unbound, and it is important to characterize this fraction.
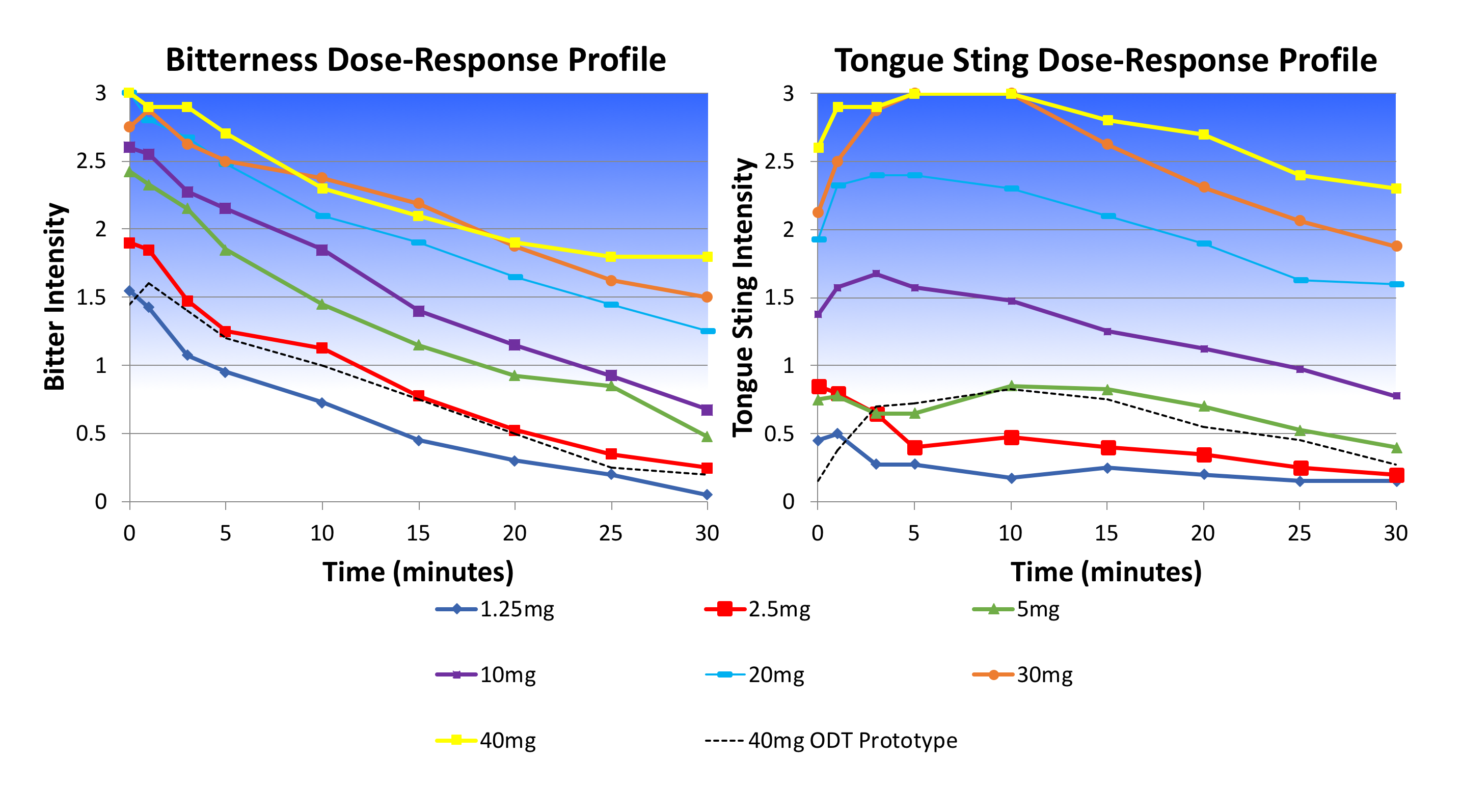
Senopsys Approach: Senopsys conducted dose-response sensory analysis to establish the maximum free-API concentration that could be effectively delivered in a palatable ODT*. Following an appropriate experimental design, the company’s trained adult sensory panelists evaluated a dilution series of API aqueous solutions. Based on the team’s understanding of recognition thresholds and the upper limitations of excipient systems in reducing aversive sensory attributes, an estimate of the maximum free-API concentration was established. The client selected adsorption as the preferred approach and working with the ODT technology provider, used this upper limit to guide formulation optimization. Promising prototypes were submitted to Senopsys for confirmation testing.
Results: Adsorption proved to be effective at reducing, but not eliminating, the aversive sensory attributes of the API. Shown below are the dose-response results for two of the aversive attributes – bitterness and tongue sting – and their corresponding intensities in an unsweetened/unflavored ODT prototype at a clinically relevant dosage strength. The remaining bitterness can be further reduced to patient-imperceptible levels through the judicious use of appropriate sweeteners, buffers, taste modifiers, flavoring aromatics and extenders, and sensate materials.
* Study conducted in accordance with Good Clinical Practice