Designing Medicines for Ease-of-Swallowing
Challenge
It is widely recognized that young children cannot swallow traditional tablets, but difficulty in swallowing tablets (dysphagia) is not limited to children. An estimated 40% of adults report difficulty in swallowing tablets1, which is increasingly common among older patients. Senopsys’ formulation and sensory scientists collaborate with pharma companies to optimize dosage forms for ease-of-swallowing, palatability and human factors.
Designing Medicines for Ease-of-Swallowing
Regulations in the EU and US require the development of formulations suitable for dosing children from birth to adolescence. As young children cannot swallow traditional tablets and capsules, alternative dosage forms are required, such as:
- Liquids – solutions, suspensions
- Chewable tablets
- Orally disintegrating (“fast dissolving/melting”) tablets
- Multiparticulates – granules, beads, mini-tablets
- Orodispersible films
But children are not the only the only ones who suffer from difficulty of swallowing, a condition known as dysphagia. Dysphagia is common among older adults as well as patients with certain neurological and degenerative disorders.
What we have done for others
Senopsys has assessed ease-of-swallowing of a variety of dosage forms. Three case examples are described below – mini-tablets, traditional film coated tablets and capsules.
Case Study #1: Assessment of Ease-of-Swallowing of Mini-Tablets in Food Dosing Vehicles
Multiparticulate dosage forms – granules, beads, mini-tablets – are increasingly under investigation as alternatives for pediatric (and other) patients who have difficulty swallowing traditional tablets and capsules. There are many form and functional properties of multiparticulates that need to be considered to ensure successful dose administration, including:
- Size. Sponsors point to FDA guidance on “sprinkle” size2; however, the guidance was based on adult swallowability studies. Mastication is a complex physiological process that enables food to be size-reduced and swallowed. It requires coordination of bones, muscles, teeth and soft tissues – all of which change during physiological development.
- Quantity. Studies to assess the suitability of mini-tablets for pediatric dosing generally compare ease-of-swallowing of 1 or 2 mini-tablets (MTs) to a small liquid volume (3-5mL), without regard to dose equivalency. It’s not uncommon for pediatric doses to require the administration of 10 or dozens on MTs.
- Functional Coating. Oral solid dosage forms may be coated for a variety of reasons – aesthetics, controlled release, ease-of-swallowing or taste masking. Importantly, the properties and performance of multiple coated mini-tablets is markedly different than a single tablet. MTs need to maintain structural integrity during dose preparation and administration to ensure proper drug release and stability. Depending on the surface properties, mini-tablets may agglomerate into a large mass in the saliva (or dosing vehicle) increasing the propensity of the patient to chew for particle size reduction. Unlike most liquids, mini-tablets may adhere to gums, teeth, and palate thereby increasing residence time in the oral cavity and sustained release of API bitterness, for example. In addition, mucosal adhesion of MTs in the throat may trigger coughing or choking (if lodged further down the alimentary canal).
- Food Dosing Vehicles. EMA guidance dictates that dosing in foods should not be relied on to improve the palatability of medicines: “the palatability of a pediatric preparation should be satisfactory on its own merit, i.e. without mixing with food or drinks”. 3 Most “pediatric-friendly” foods do not have sufficient flavor intensity to mask the aversive taste attributes of most drug actives, resulting in an unpalatable dosing experience. As food preference is a learned behavior, an unpalatable dose risks the creation of food aversions, which could be practically harmful if dosing in breast milk or formula. Foods also vary greatly in terms of composition and chemical properties (pH, fat and sugar content, water activity, viscosity and other physical properties). All of these can have a dramatic effect on API release, stability, and palatability.
Senopsys has conducted multiple human factor / sensory studies of MTs in food dosing vehicles to measure ease-of-preparation, ease-of-administration, palatability and ease-of swallowing. One such study measured the performance interaction between selected dosing vehicle and coated mini-tablets.
Approach
A fixed number of mini-tablets were evaluated in 47 food dosing vehicles. A panel of trained sensory panelists measured each food/tablet combination across 7 sensory attributes.
Results
Senopsys found that dosing vehicles with lower tendency-to-chew and less oral cavity adhesion were most strongly correlated to overall ease-of-swallowing. Mechanistically, these attributes are those related to the mastication process in the oral cavity. Sensory attributes related to mechanics in the throat (e.g. esophageal adhesion) were less related to ease-of-swallowing but indicate a potential for discomfort or to induce a cough response.
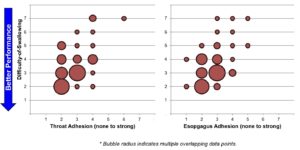
Conclusion
Even with identical coated tablets, the functional properties of the dosing vehicle can have significant impact on the overall swallowability of the dose.
Case Study #2: Assessment of Ease-of-Swallowing of Film Coated Tablets to Guide Formulation Optimization
As described, patients have difficulty swallowing solid oral dosage forms due to physiological or psychological (experience based) factors. Additionally, the esophageal transit time of film-coated tablets has been reported to be shorter than that of uncoated tablets; though tablet size and shape are also contributing factors4,5. In the following study, a film coating excipient supplier was developing new formulations to improve the “swallowability” of film coated tablets.
Approach
Six different tablet film coating prototype formulations and an uncoated comparator were measured by a trained human sensory panel for swallowability. First, the panelists trained on a series of commercial tablets and capsules using the Flavor Profile Method6, a standard ASTM/ISO method of sensory analysis. By this method, a standardized lexicon was established to identify and define relevant texture and mouthfeel attributes and evaluation protocols. Next the sensory panelists measured the above coated prototype tablets using each of these identified sensory attributes as well as overall ease-of-swallowing. The attributes were measured during three phases of dose administration – initial roll of the tablet in the oral cavity, upon swallowing and after swallowing.
Results
As shown below, certain mouthfeel characteristics were measured that were most correlated with ease-of-swallowing. Tablets perceived as more smooth, slippery, and lowest in throat irritation were most strongly correlated to high ease-of-swallowing. Other measured attributes (e.g. tablet stickiness/tackiness) were not correlated.
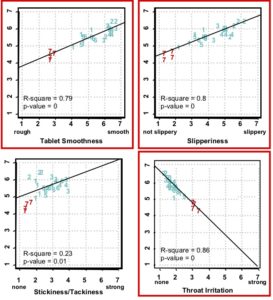
Conclusion
Tablet coatings can be optimized for ease-of-swallowing applying experimental design of coating systems with sensory panel measurement.
Case Study #3: Ease-of-Swallowing of Different Size Capsules
For a chronic condition with multiple daily dosing of large capsules, a developer hypothesized that their new capsules were easier to swallow than their competitor’s due to their smaller size (~30%) and different gelatin matrix. If proved true, the developer believed its product would have higher patient acceptability and compliance.
Approach
Ease-of-swallowing was measured in healthy adult volunteers (N=100) using a seven-point scale ranging from extremely easy to extremely difficult to swallow.
Results
Though the prototype capsules were directionally easier to swallow, the mean ease-of-swallowing values were not found to be significantly different.
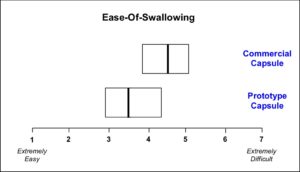
Conclusion
Ease-of-swallowing may not be directly proportional to capsule size, and/or this change in a gelatin matrix was insufficient to achieve statistical superiority against the reference commercial capsule.
Designing Medicines for Ease-of-Swallowing — A Recap
Regulations require developers to demonstrate the palatability of an age-appropriate drug product formulation in pediatric patients. Historically, liquids (ready-to-use or powders for constitution) have been the standard dosage form for children, permitting dose titration. Today there’s increasing interest in oral solid dosage forms that do not contain or require water, while maintaining dose flexibility. Multiparticulates – granules, beads, mini-tablets.
Some patients, both young and old, have difficulty swallowing solid oral dosage forms. Mastication and swallowing are complex physiological processes that are highly dependent on development stage (age). They are also influenced by the physical and mechanical properties of the dosage form and delivery vehicle. Additional human factors issues associated with dose preparation and administration need to be considered. The drug product/delivery system also needs to be palatable to ensure it’s not rejected prior to swallowing.
Senopsys has been helping clients address these and other issues since our founding. We welcome the opportunity to share our experience with companies interested in designing medicines for both ease-of-swallowing and palatability.
References
1 Chang RK, Guo X, Burnside B, Couch R. Fast-dissolving tablets. Pharm Technol N Am. 2000;2452- 58
2 Food Drug Administration Center for Drugs Evaluation Research (2012). Guidance for Industry: Size of Beads in Drug Products Labeled for Sprinkle (FDA Maryland).
3 European Medicines Agency Committee for Medicinal Products for Human Use (2013). Guideline on pharmaceutical development of medicines for paediatric use (EMA London).
4 Perkins, A.C., Wilson, C.G., Frier, M., et. al. The use of scintigraphy to demonstrate the rapid esophageal transit of the oval film-coated placebo risedronate tablet compared to a round uncoated placebo tablet when administered with minimal volumes of water. Int. J. Pharm. 2001; 222: 295-303.
5 Channer, K.S., Virjee, J.P. The effect of surface coating of tablets on oesophageal transit. Brit. J. Pharm. Pract. 1985; Jan: 9-14.
6 Keane, P. 1992. The flavor profile. In ASTM Manual on Descriptive Analysis Testing for Sensory Evaluation (R.C. Hootman, ed.) pp. 5–15, American Standards for Testing and Materials, Philadelphia, PA.

