The Role of Sweeteners in Taste Masking

Sweeteners play an important role in developing palatable, taste-masked formulations. They can help reduce bitterness but are most effective when combined with buffers for sourness and sodium salts for saltiness.
Available Sweeteners
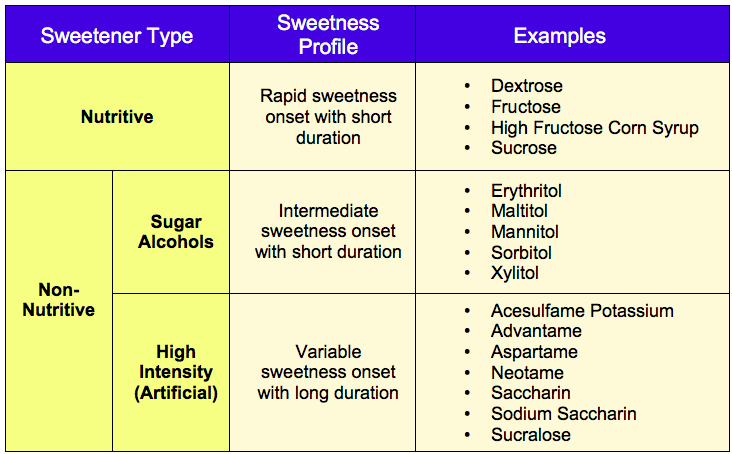
There are a multitude of sweeteners available to the pharmaceutical scientist. Sweeteners may be grouped into two categories – nutritive and non-nutritive. Nutritive sweeteners deliver calories and as the name suggest non-nutritive do not. Non-nutritive sweeteners can be further characterized as bulk (sugar alcohols) and high intensity (artificial). Some excipients that are sweet are often incorrectly listed in publications as “sweeteners.” These are excipients used primarily for purposes other than imparting sweetness, for example, co-solvents such as glycerol or propylene glycol, and bulking agents such as lactose or maltodextrin. The most common examples of excipients used for the purpose of sweetening drug formulations are shown below.
Sweetener Selection
The selection of candidate sweeteners is informed by technical, regulatory and clinical considerations.
Technical Considerations
There are numerous technical issues that need to be considered in identifying candidate sweeteners for the formulation design space. These include:
- Sweetness Profile. The sweetness profile (intensity, onset and duration) of each sweetener is different as summarized in the table above. Applying the concepts of taste/taste interaction, the intensity and duration of the aversive attribute (bitter, salty or sour) informs identification of candidate sweeteners. There’s much more practical information on sweetener intensity, onset and duration to guide selection that will be covered in future posts.
- Physical Properties. Nutritive sweeteners and sugar alcohols have low relative sweetness and are sometimes employed as bulking agents in dosage forms requiring additional mass, such as chewable tablets and powder sachets. However, due to their relatively modest sweetness, many formulations require combinations of bulk and high intensity sweeteners to provide the requisite sweetness profile. Some bulk sweeteners have added benefits (e.g. improved freeze/thaw stability) and disadvantages (e.g. tendency to crystalize on bottle cap threads of multi-dose containers) that should also be taken into consideration.
- Microbiologic Stability. Depending on usage level, bulk sweeteners can improve microbiological stability by lowering water activity, reducing the need for chemical preservatives.
- Chemical Stability. The artificial sweeteners typically exhibit good solid-state stability across the board, but some have poor stability in solution. For example, aspartame readily hydrolyzes in aqueous environments, and a liquid aspartame-sweetened product would experience drastic changes by 6 months storage. Some manufacturers do not recommend that sucralose be used in formulations with a pH above 7.
Regulatory Considerations
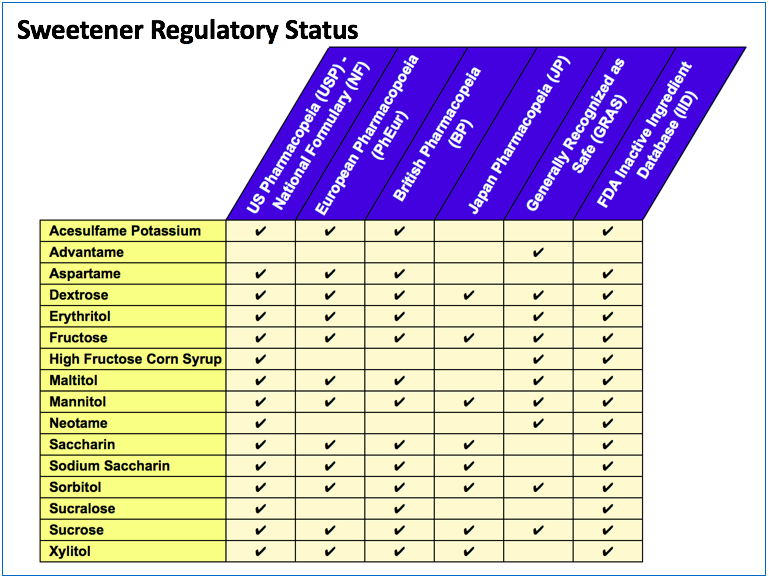
The regulatory acceptability of sweeteners can vary by geography. Many of the common sweeteners are listed in the various pharmacopeia – United States Pharmacopeia and National Formulary (USP-NF), British Pharmacopeia (BP), Japan Pharmacopeia (JP), European Pharmacopoeia (PhEur). Some have been determined to be Generally Recognized as Safe (GRAS) by the manufacturer, or by the Flavor and Extract Manufacturers Association (FEMA), in accordance with the 1958 Food Additives Amendment to the Federal Food, Drug and Cosmetic Act. Additionally, the FDA maintains the Inactive Ingredient Database (IID) which lists excipients with prior precedent of use in approved pharmaceutical formulations.
A high level summary of the regulatory status of the common sweeteners is shown below.
Other regulatory considerations include a label warning for phenylketonurics of the phenylalanine content of aspartame-sweetened products.
Clinical Considerations
Clinical considerations may weigh against the use of nutritive sweeteners due to co-morbidity issues (e.g., diabetes) and their cariogenic potential, though the latter is often overstated, particularly for life saving/extending drugs. Sugar alcohols can increase gastrointestinal motility but at typical usage levels, this is generally only a concern in very young patients or in special populations such as those undergoing Total Parenteral Nutrition (TPN).
The Importance of Sweeteners
For most APIs, creating a proper white base formulation is the most critical aspect of taste masking, and the sweetener system plays a crucial role. Many excipients are sweet; but only a subset has the primary function of imparting sweetness to the formulation, i.e., are true sweeteners. The selection of sweeteners is informed by a myriad of technical, regulatory and clinical considerations. From a taste masking perspective, the sweetness profile – intensity, onset and duration – is critically important. Get it right and prospects for palatability are good. Get it wrong and the resulting formation may actually taste worse!
Taste Masking Challenge? Senopsys Can Help!
Are you faced with the need to develop a palatable drug product to support clinical trials or commercial development? Our scientists are expert in both taste assessment and taste masking.
We use our experienced GCP-compliant taste panels and analytic tools to quantify the taste masking challenge and guide formulation development. And we apply a structured, sensory-directed development approach pioneered in the food industry to create palatable, taste-masked drug formulations for liquids, powders and solids.

