Pediatric Investigation Plans: Part 1 – Determining Taste Masking Challenge

Ensuring drug palatability is critically important for the development of pediatric medicines. Both FDA and EMA require Pediatric Investigation Plans / Pediatric Study Plans (PIP / PSP) that address acceptability, but do not suggest exactly how this should be performed. Case in point, EMA’s Guideline on Pharmaceutical Development of Medicines for Pediatric Use (2014) specifies that the “evaluation of the patient acceptability of a pediatric preparation should be an integral part of the pharmaceutical and clinical development.” But does not prescribe any methods for palatability/acceptability testing. Rather, EMA relies on the applicant to specify the choice of methods and acceptance criteria, and justify their use. Though the guideline suggests that the taste of the active substance should contribute to the choice of finished dosage form(s) and route(s) of administration, most published decision trees use the criterion of “acceptable taste” rather than a quantitative measure of the taste masking challenge of the drug active.
The logic is circular.
Acceptability is what consumer researchers term an “affective” test, which is used to measure preference or liking of a final formulation. “Analytical” tests are used to quantitatively measure product attributes and guide formulation development – in the case of drug development to measure the taste masking challenge. In other words, first the taste masking challenge should be determined analytically (to guide formulation development), and acceptability should be measured afterwards; once the formulation is finalized.
Analytical and affective sensory testing methodologies trace their origins to the food industry, as we have described previously. Today the methods are well established and accepted by standard setting organizations, e.g., ASTM and ISO, but are largely unfamiliar to pharmaceutical scientists as their primary use is in the food industry.
Framework for Developing Palatable Drug Products
In 2013, the AAPS Pediatric Formulations Task Force published their report on pediatric medicines. The manuscript included a decision-tree framework that identifies the sequence of questions that need to be answered to guide formulation development. It is built on the adage that you can’t improve what you can’t measure.
The flowchart is divided into four Stages:
- Taste Assessment
- Technology Guidance
- Formulation Guidance
- Clinical/Commercial Development
The first Stage is supported by Analytical testing methodologies which inform the selection of dosage form and taste masking approach. This stage also form the basis of Senopsys’ FlavorMetricsSMTaste Assessment platform.
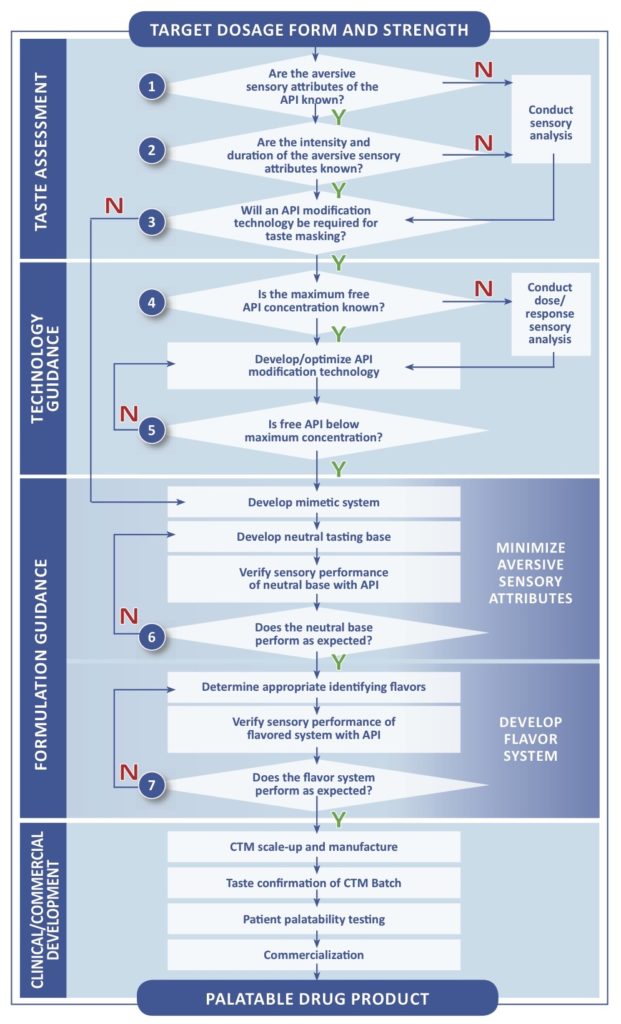
In this post we focus on the first section of the decision tree, Taste Assessment, which encompasses the initial study necessary to quantify the taste masking challenge of the drug active. This is a necessary first step in planning an effective pediatric development program described in Pediatric Investigation Plans / Pediatric Study Plans.
Taste Assessment
A taste assessment informs the key questions that need to be answered to support development of age-appropriate, palatable drug products.
Question 1: Are the aversive sensory attributes of the of the API known?
Consumers and patients have difficulty accurately describing their perceptions. For example, sour and bitter basic tastes are often confused. Untrained healthy volunteers often describe drug as “bad” or “yucky” tasting, which is not very helpful as there’s no “good” or “yummy” ingredient on the shelf to improve palatability!
Senopsys compiled data from one hundred taste assessment studies and the results are summarized in the pie chart below. More details can be found here.
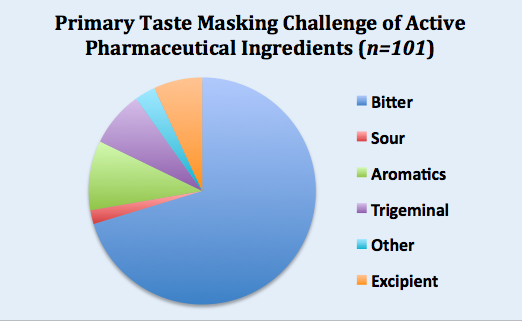
The primary taste masking challenge for 75% of the APIs was bitterness, followed by obnoxious odors (aromatics) at 10%, and trigeminal irritancy (e.g., burning) at 8%. Interestingly, 7% of APIs were fundamentally tasteless, but their challenge was driven by the aversive attributes of the excipients (e.g., solubility enhancers).
In summary, not all APIs are created equal. As the formulation strategies for mitigating bitterness, malodor, irritation and texture differ, it’s important to correctly identify the aversive attributes at the outset.
Question 2: Are the intensity and duration of the aversive attributes known?
In addition to correctly identifying the aversive sensory attributes, it’s important to understand their strength and temporal characteristics. From a taste-masking perspective, it’s much more difficult to mitigate the effects of strong-intensity aversive attributes that linger for a long time than those that fade quickly.
Senopsys uses the open source Flavor Profile method to quantify the sensory attributes of drug actives and formulations. The output of Flavor Profile Analysis is analogous to a chromatogram, wherein each peak represents a separately identified sensory attribute and the peak height/area corresponds to perceived strength or intensity of each attribute.
Question 3: Will an API modification technology be required for taste masking?
Sensory time-intensity profiles provide an indication of the overall taste masking challenge. The figure below shows the bitterness profiles of four different APIs. Based on an understanding of the sensory dose/response function and the limitations of excipients in reducing aversive sensory attributes, color-coded decision boundaries can be overlaid.
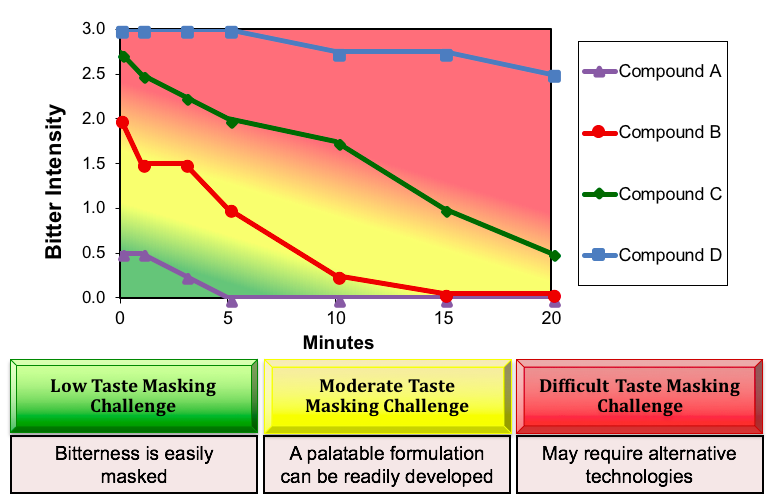
For many APIs, a traditional excipient approach to taste masking may be sufficient to develop a palatable drug product, i.e., through the use of sweeteners, buffers, taste modifiers and flavors. Other APIs, such a Compound D, need to be “sequestered” from the taste receptors using particle coating, adsorption or other technology to yield a palatable drug product.
When a taste masking technology such as particle coating or adsorption is required, additional questions need to be answered to guide development as highlighted in the “Technology Guidance” section of the flowchart. These questions will be explored in Part Two of this series.
PIP Strategy
In the absence of clear regulatory guidance, it’s no surprise that applicants are filing PIPs late and filling them with extraneous unnecessary CMC information that does not address the health authority’s questions in an attempt to avoid what could be costly commitments.
What’s the alternative?
Savvy applicants propose a quantitative sensory analysis of their active using approved methodologies as a Key Quality-related study element to address taste masking and palatability (PIP Template Section D.2.2). For maximum benefit, these studies should be conducted immediately following successful first in human studies, e.g., single ascending dose (SAD) and multiple ascending dose (MAD) studies. This enables the applicant to drive the process by having both qualitative and quantitative data to develop a cogent and achievable plan to propose in the PIP.
These are the same elements of the Senopsys’ FlavorMetricsSMplatform, and the benefits are compelling:
- Characterize the aversive attributes of the API – ensure the correct problem is being solved.
- Quantify the taste masking challenge – inform dosage form selection and taste masking approach.
- Guide dosage form selection – leverage qualitative and quantitative sensory analysis data.
- Accelerate development – initiate necessary taste masking technology development/optimization early.
- Reduce risk – avoid costly and unnecessary commitments by having science-based justification.
In summary, regulatory agencies take a “propose and justify” approach to PIPs/PSPs. While many may see this as a problem, we see this as an opportunity to lead from a position of strength.
Taste Masking Challenge? Senopsys Can Help!
Are you faced with the need to develop a palatable drug product to support clinical trials or commercial development? Our scientists are expert in both taste assessment and taste masking.
We use our experienced GCP-compliant taste panels and analytic tools to quantify the taste masking challenge and guide formulation development. And we apply a structured, sensory-directed development approach pioneered in the food industry to create palatable, taste-masked drug formulations for liquids, powders and solids.


And if anyone asks, that’s my daughter Zoe in the air.