It is widely understood by physicians, caregivers, and industry experts that medicines must be palatable to ensure dose acceptance and compliance.
Many drug substances are bitter, some extremely so, or have other “negative” or aversive sensory characteristics such as an unpleasant aromas or mouth irritation. As a consequence, the development of palatable drug products can be a daunting challenge.
The food industry strives to create products that “delight the palate” as flavor quality drives sales. Medicines, on the other hand, are developed for efficacy and safety. Palatability is necessary to ensure dosing compliance, rather than to promote consumption, and is therefore of secondary importance.
Palatable drug products are those in which the aversive sensory attributes have been minimized or eliminated. In other words they are not overly bitter, produce little trigeminal irritation, are smooth not gritty and have no perceptible malodors. It is an industry myth that flavor preference – orange, grape, chocolate or mint – is the primary determinant of palatability. In actuality it’s the degree to which the negative attributes have been eliminated, that’s the true measure of palatability.
Senopsys is a unique specialty service provider to the pharma industry, conducting both taste assessment of APIs and taste masking of drug products using GCP-compliant human taste panels, following internationally recognized analysis methods originally developed by the food industry.
The taste masking challenge of an API is a function of its time-intensity profile. The stronger the intensity and
longer the duration of bitterness, or other aversive attributes, the greater the challenge.
Quantify the taste making challenge of your API early in the development process.
The FlavorMetrics℠ Bitterness Profile provides quantitative data to inform formulation development.
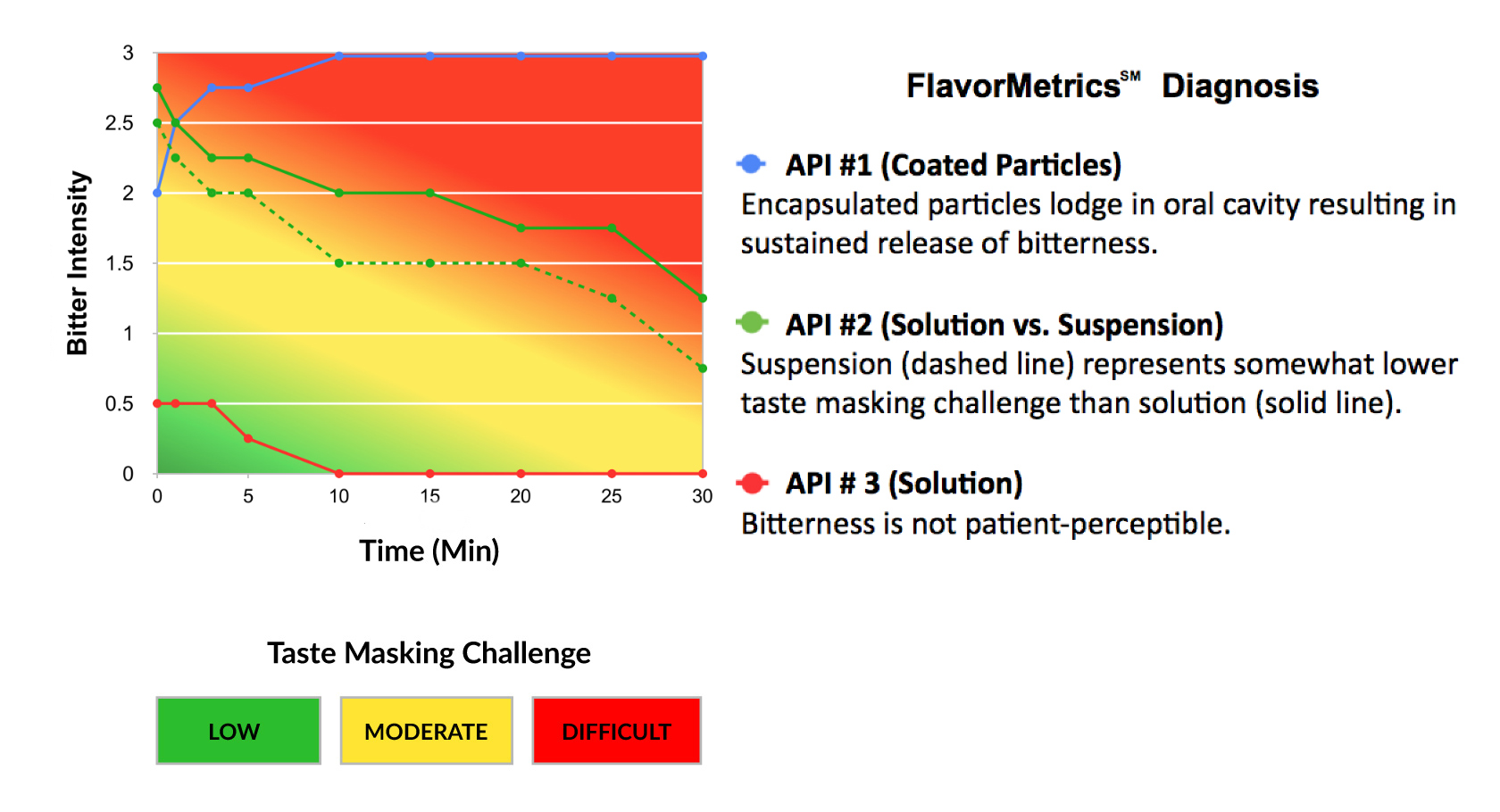
Select the taste masking technology that is most appropriate for your formulation
Dose/response sensory analysis of APIs generates quantitative data to enable the selection, development and optimization of taste masking technology.
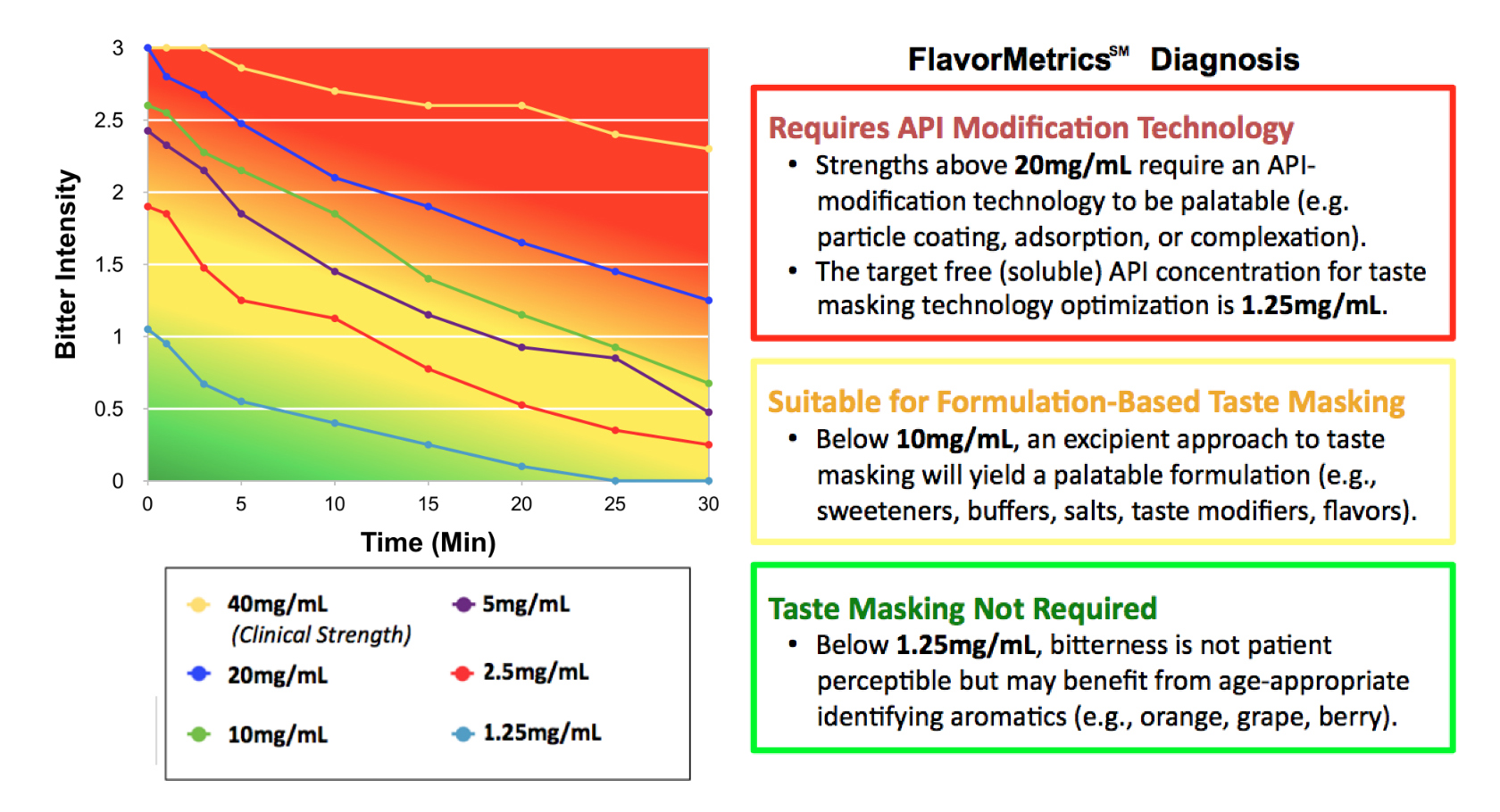
Measure the flavor quality of prototypes and competing products.
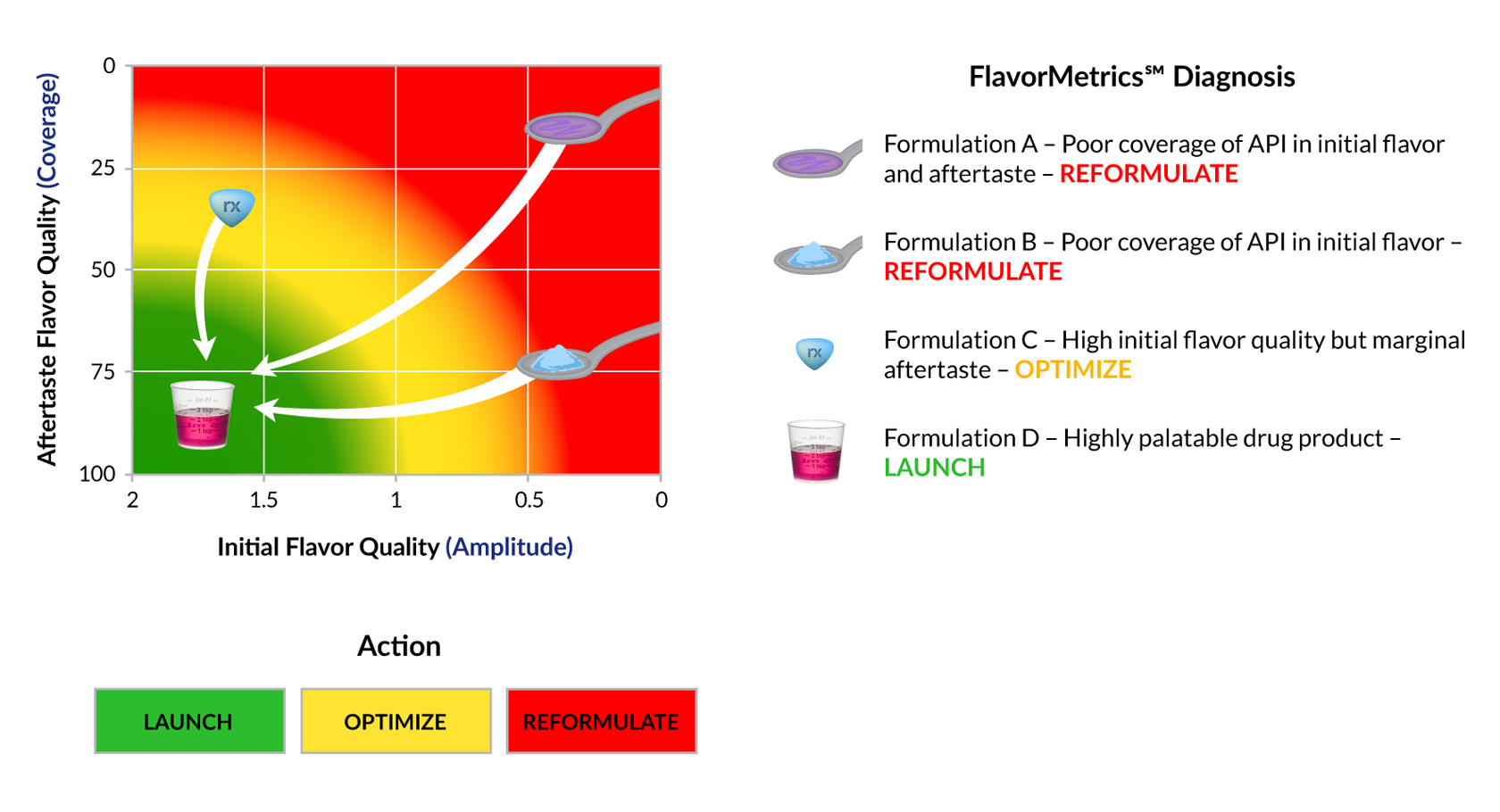
Patient acceptability of a drug product is a function of both its initial taste characteristics and the aftertaste it leaves behind. Both must be addressed otherwise palatability suffers. When palatability suffers so do patient adherence, health outcomes and product sales.
The FlavorMetrics℠ Palatability Profile is an empirical model that relates the quality of the initial flavor and aftertaste. It can be used to:
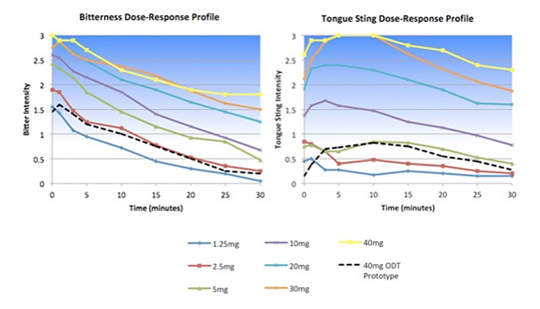
The client was developing an orally disintegrating tablet (ODT) form of an approved drug known to be extremely bitter. Senopsys conducted dose-response sensory analysis to establish the maximum free API concentration that could be effectively delivered in a palatable ODT and to evaluate the taste masking effectiveness of adsorption technology.

How good is your knowledge of taste masking? Learn the truths behind the science and what it means for your development program.
Senopsys is the taste-masking development partner of choice for 15 of the top 25 global pharma companies as well as dozens of emerging and mid-size pharma and biotech companies.
Ask us questions, inquire about our services or schedule a “lunch and learn” seminar about the art and science of taste masking.